Abstract
Introduction: Prophylactic replacement of coagulation factor VIII (FVIII) is the standard of care in hemophilia A, given its natural mechanism of action, strict homeostatic regulation, and consistent safety profile. Recombinant FVIII Fc fusion protein (rFVIIIFc) is an extended half-life therapy that demonstrated safety and efficacy in previously treated pediatric, adolescent, and adult subjects with severe hemophilia A in the Phase 3 A-LONG and Kids A-LONG trials (NCT01181128 and NCT01458106, respectively) (Young et al, J Thromb Haemostas, 2015; Mahlangu et al, Blood, 2014). Herein, the final results are reported from ASPIRE (NCT01454739), the long-term extension trial of those 2 studies.
Methods: This was an open-label, multicenter, long-term trial of previously treated subjects of all ages with severe hemophilia A. Subjects received 1 of the 4 following regimens: individualized prophylaxis (IP; rFVIIIFc 25‒60 IU/kg every 3-5 days, or twice weekly), weekly prophylaxis (WP; rFVIIIFc 65 IU/kg every 7 days), modified prophylaxis (MP; personalized dosing), or episodic treatment (ET; on-demand dosing based on bleeding episodes). Subjects <12 years of age were eligible for only IP or MP. Investigators could switch a subject's treatment group at any time; therefore, each subject may appear in >1 treatment group. The primary endpoint was development of inhibitors. Secondary endpoints included annualized bleeding rates (ABRs), joint ABRs, spontaneous joint ABRs, exposure days (ED), and factor consumption. Descriptive statistics were used for analysis. Analyses were performed separately based on parent study.
Results: A total of 211 subjects (150 were from A-LONG and 61 were from Kids A-LONG) enrolled in ASPIRE, and 186 subjects (132 from A-LONG and 54 from Kids A-LONG) completed the study. Subjects from Kids A-LONG had a median (range) age of 6.0 (2-12) years. Of the subjects from the A-LONG study enrolled to ASPIRE, the median (range) age was 31.0 (13-66) years. Most subjects received IP regardless of parent study (Kids A-LONG: IP [n=59], MP [n=3]; A-LONG: IP [n=110], WP [n=27], MP [n=21], ET [n=13]).
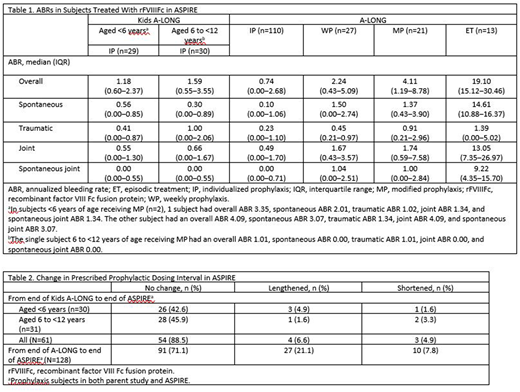
No inhibitors were observed throughout the study, and the overall safety profile of rFVIIIFc was consistent with the parent studies and prior interim analyses. ABRs remained low throughout the entirety of ASPIRE in subjects prescribed IP (Table 1). For subjects from the Kids A-LONG study, the median (range) number of ED during ASPIRE was 332.0 (18.0-467.0) days. Total median (range) treatment duration was 166.6 (14.4-203.0) weeks. The median (range) number of ED for A-LONG subjects was 267.5 (8.0-660.0) days. Total median treatment duration was 201.4 (5.1-274.6) weeks. Overall, the median (range) duration of treatment with rFVIIIFc was 4.1 (0.4, 5.9) years.
From the end of the parent study to the end of ASPIRE, the rFVIIIFc dosing interval increased for 6.6% and 21.1% of subjects from Kids A-LONG and A-LONG, respectively (Table 2). There was no change in median weekly factor consumption from the end of either parent study to the end of ASPIRE. For subjects from Kids A-LONG, the median (IQR) was 0.0 (0.0‒3.2), while the median (IQR) for those from A-LONG was 0.0 (0.0‒0.0).
Conclusions: Throughout up to 4 years of treatment with rFVIIIFc in the ASPIRE extension study, no inhibitors were reported, and low ABRs and extended dosing intervals were sustained. These data are consistent with the well-characterized safety profile and durable efficacy of rFVIIIFc prophylaxis in previously treated subjects of all ages with hemophilia A.
Nolan:Bayer: Research Funding; CSL Behring: Research Funding; Sobi: Research Funding. Mahlangu:Sanofi: Research Funding, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau; Alnylam: Consultancy, Research Funding, Speakers Bureau; Bayer: Research Funding; Biogen: Research Funding, Speakers Bureau; Chugai: Consultancy; Catalyst Biosciences: Consultancy, Research Funding; Amgen: Consultancy; Biomarin: Research Funding, Speakers Bureau; CSL Behring: Consultancy, Research Funding, Speakers Bureau; NovoNordisk: Consultancy, Research Funding, Speakers Bureau; LFB: Consultancy; Shire: Consultancy, Research Funding, Speakers Bureau; Sobi: Research Funding, Speakers Bureau; Spark: Consultancy, Research Funding. Young:Bayer: Consultancy; Bioverativ: Consultancy, Honoraria; Kedrion: Consultancy; Novo Nordisk: Consultancy, Honoraria; CSL Behring: Consultancy, Honoraria; Genentech/Roche: Consultancy, Honoraria; Shire: Consultancy, Honoraria. Konkle:Sangamo: Research Funding; Gilead: Consultancy; Spark: Consultancy, Research Funding; BioMarin: Consultancy; CSL Behring: Consultancy; Genentech: Consultancy; Bioverativ: Research Funding; Pfizer: Research Funding; Shire: Research Funding. Pasi:Shire: Speakers Bureau; Alnylam: Honoraria, Research Funding; Bayer: Speakers Bureau; Octapharma: Honoraria; Pfizer: Speakers Bureau; Biomarin: Honoraria, Research Funding; Sobi: Honoraria; Bioverativ: Honoraria, Research Funding; NovoNordisk: Speakers Bureau; Catalyst Bio: Honoraria; Apcintex: Honoraria. Oldenburg:Novo Nordisk: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Biotest: Honoraria, Research Funding; Biogen: Honoraria, Research Funding; Shire: Honoraria, Research Funding; Grifols: Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Octapharma: Honoraria, Research Funding; CSL Behring: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Swedish Orphan Biovitrum: Honoraria, Research Funding. Nogami:Chugai Pharmaceutical Co., Ltd: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Anti-FIXa/X bispecific antibodies , Research Funding, Speakers Bureau. Tripkovic:Sobi: Employment. Rudin:Bioverativ: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal